

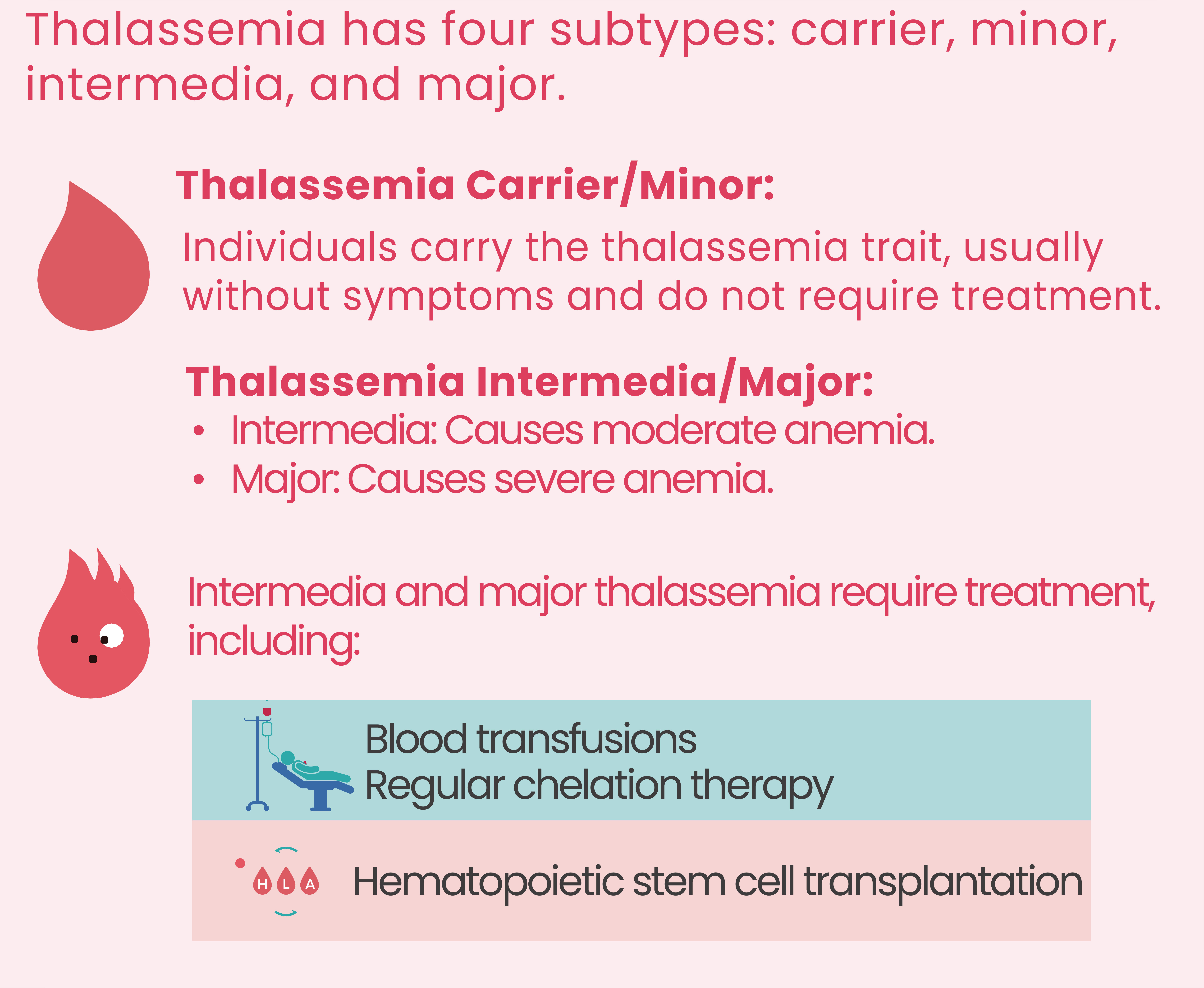
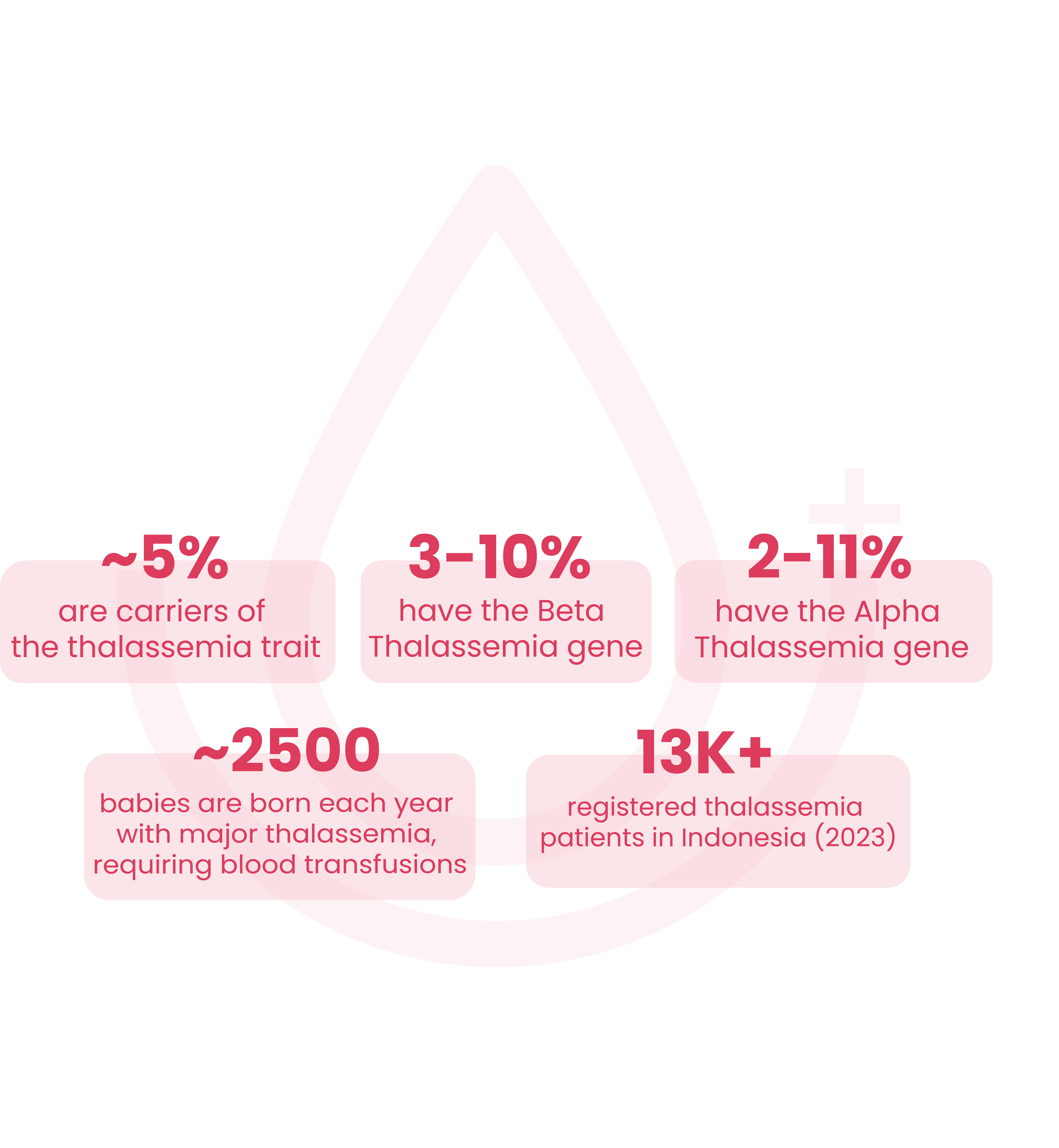
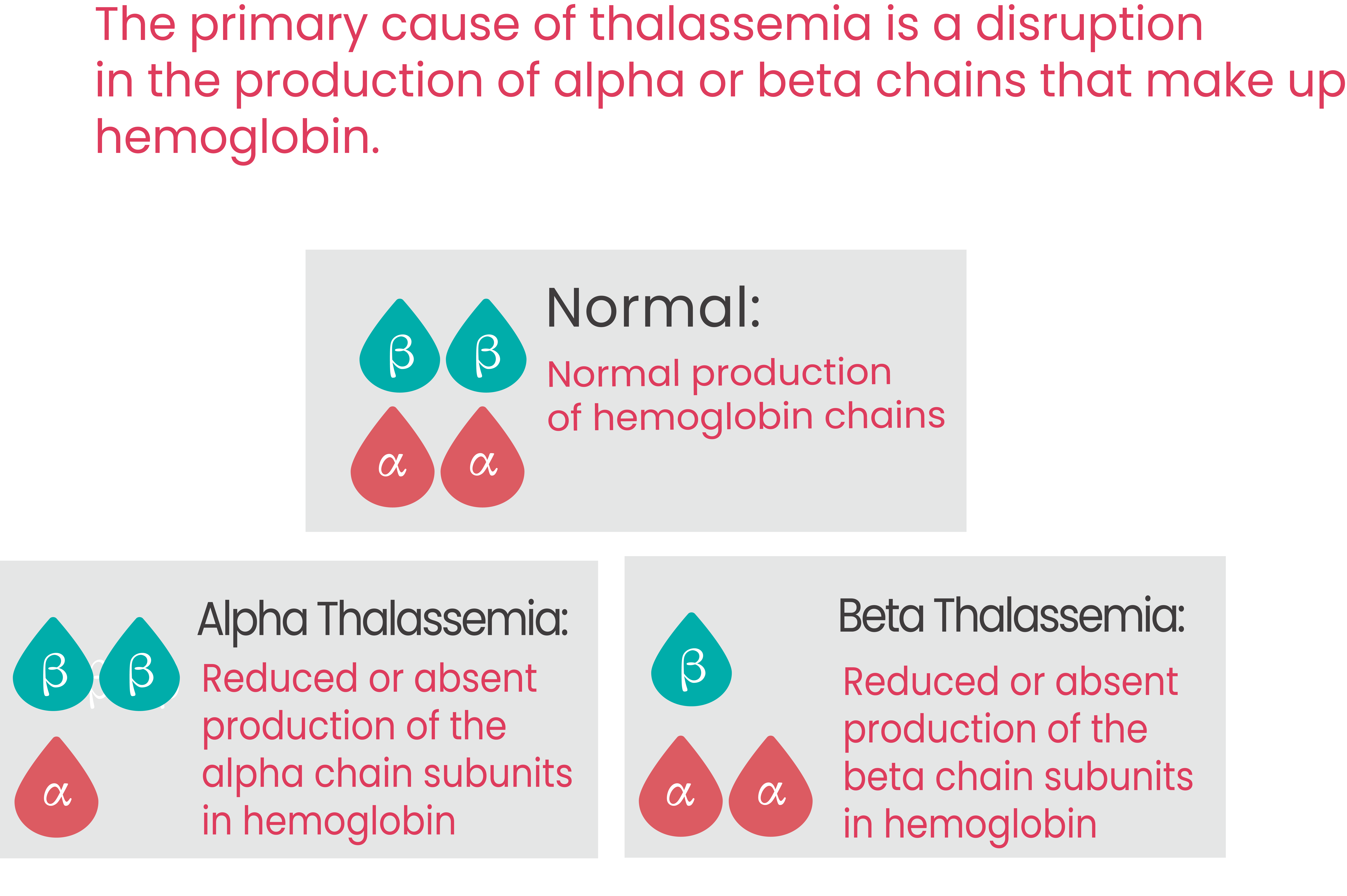
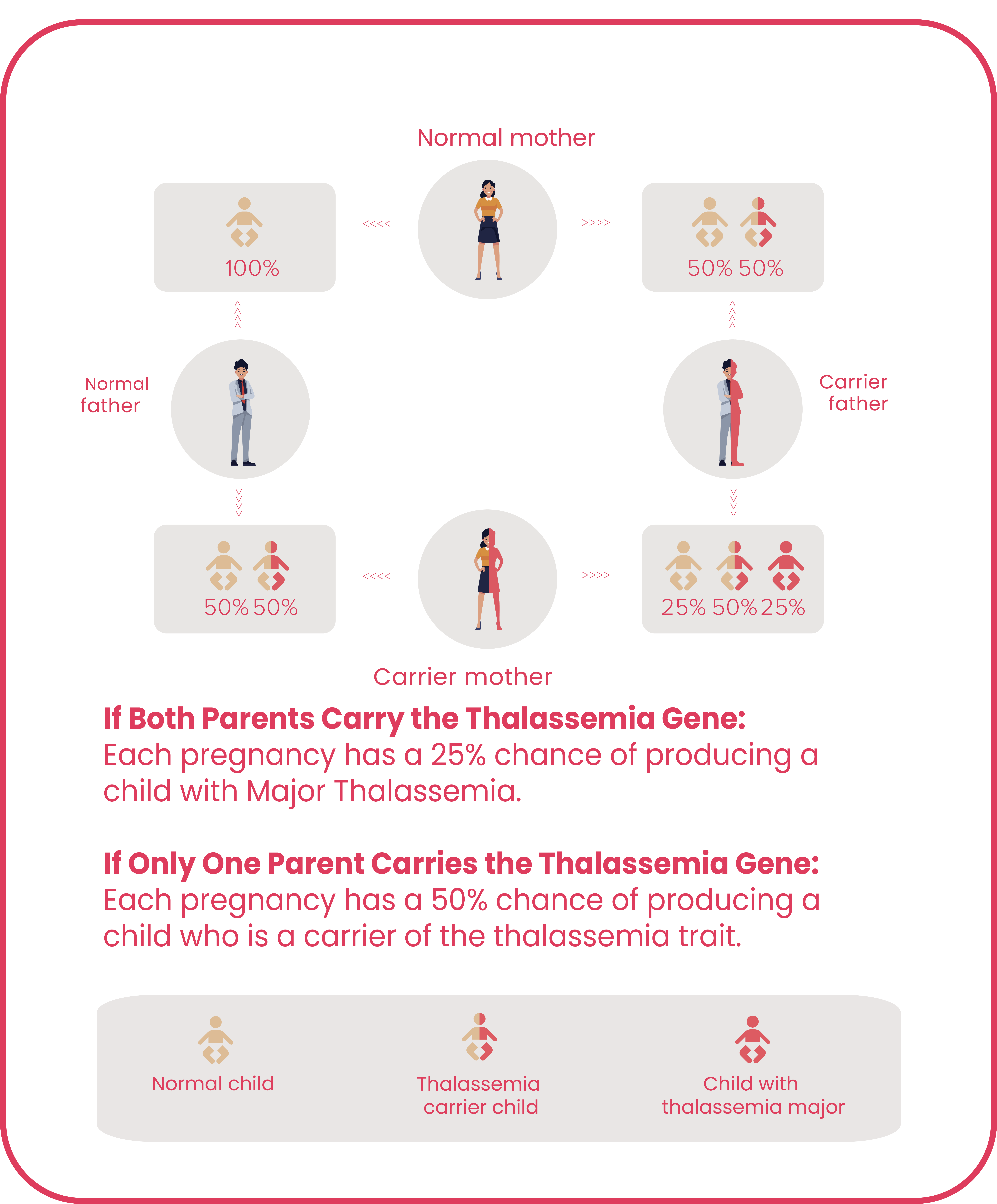
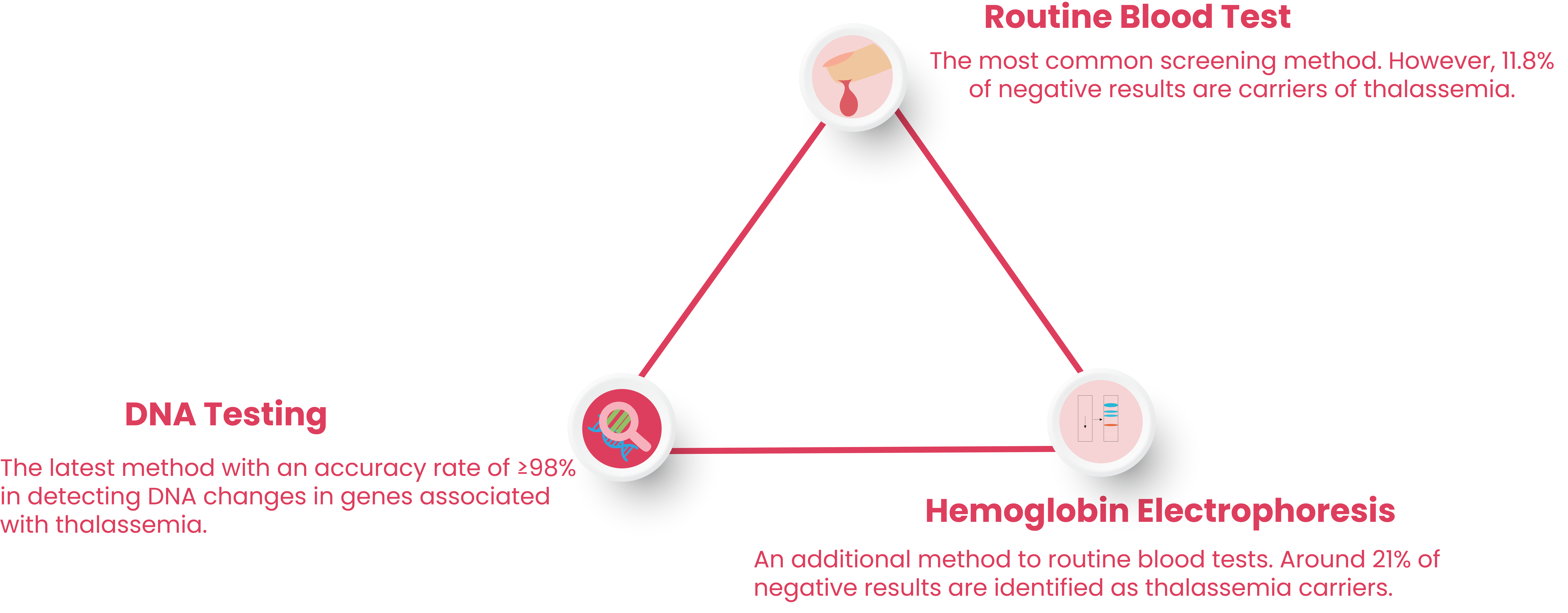
Thalassemia is a non-contagious genetic disorder caused by changes in genes that affect hemoglobin production..

Latest Technology and No.1 Indonesia
Genetic screening of Thalassemia based on
Next-Generation Sequencing and Multiplex PCR, the first and only in Indonesia

Comprehensive
Detection of more than 500 genetic variations of Thalassemia
Safe & Easy
Using 3 mL of blood sample
NALEYA – THALASEQ is intended for every individual or couple, especially:
| 4 Alpha Thalassemia Deletion | –SEA, -α3.7, -α4.2,–THAI |
| 3 Beta Thalassemia Deletion | Chinese, SEA-HPFH, Taiwan |
| >153 Alpha Thalassemia Point Mutations | Hb Westmead, Hb Quong Sze, Hb Constant Spring, Initiation codon (-T), Hb Codon 8 (-C) Alpha0, Alpha2 Codon 30 del GAG, α2 Codon 31 (AGG>AAG), Codon 40 AAG>AA-, Codon 61 AAG>TAG , Hb Phnom Penh, Hb Amsterdam-A1, Fusion gene, IVS-I-117 (G>A)… |
| >348 Beta Thalassemia Point Mutations | -90 (C>T), -88 (C>T), -72 (T>A), -31 (A>C), -29 (A>G), -28 (A>G), Initiation codon ATG>AGG, CAP +8 (C>T), Codon 5 (-CT), Codons 8/9 (+G), Codons 14/15 (+G), Codon 17 (A>T), Hb E, Hb Dhonburi, Codons 27/28 (+C), Codon 31, Codon 37 (TGG>TAG), Codon 41 (-C), Codons 41/42 (-TTCT), Codon 43 (G>T), Codons 54-58 (TATGGGCAACCCT), Codons 71/72 (+A), Codons 89-93 (-14bp): (AGTGAGCTGCACTG), IVS-I-1 (G>T), IVS-I-5 (G>C), IVS-II-5 (G>C), IVS-II-654 (C>T), Poly A (A>G)… |

Patients underwent pre-test genetic counseling and signed a consent form.

Patient sample collection and delivery to the laboratory

The process of testing samples in the laboratory using the Next Generation Sequencing and multiplex PCR methods.

Results are delivered within 20 – 30 business days.

The patient underwent post-test genetic counseling with the doctor.
© 2025 Naleya Genomics – All Rights Reserved.